Using Flash Cards to Study Organic Chemistry
There are many different ways to study organic chemistry. I’m pretty sure most of you know about, and many of you actually use this simple learning strategy—flash-cards. Now, how exactly do you make your flash cards? Do you just put some info you need to remember on it and flip through your cards from time to time? Or, maybe, you have a name of a concept on one side and some definitions on the other side? Or, when it comes to chemistry flash-cards, you put a reaction on one side and the reaction name on the other side? Did I guess right? That’s what most of the students I’ve ever worked with do. I bet, right about now you, probably, get this nagging feeling that I am about to tell you that you do it wrong. And you are right to think so—if you are doing your flash cards the way I described above, you are doing it wrong!
If you just put some info on your card and you are trying to learn the concept by flipping through your cards from time to time, you are doing it in the passive learning style. There’s nothing more dangerous for you than the passive learning. Why? Coz’ it takes for freaking EVER! Mindless repetition of things such as concepts, definitions, or reactions will eventually get you to remember those, but you’ll spend way too much time getting to that point and, let’s be honest, you don’t have a luxury of time when trying to master organic chemistry within the frames of a regular college semester.
You remember that your instructor told you that you should spend at least one hour studying for every hour spent in the lecture hall. Sounds familiar? Well, the important part there is that this studying time has to be an active studying time! Now, the problem we have is changing something about how we learn from the passive mode into an active mode.
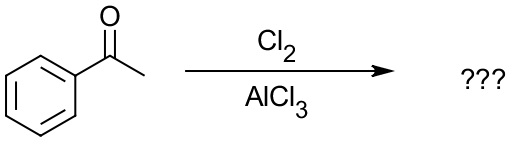
But how can that be applied to flash-cards, you ask? Let’s look at a typical Electrophilic Aromatic Substitution (EAS) reaction between acetophenone and chlorine in a presence of aluminum chloride:
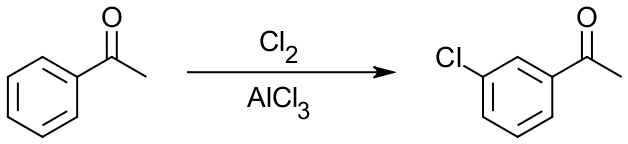
If you’re in a second semester of organic chemistry sequence, it’s one of the reactions you’re about to learn in class. So, what three main areas do we have in this reaction? We have: (1) reactants or starting materials (SM), in this case the acetophenone molecule, (2) reagents (Rg), Cl2 and AlCl3 here, and (3) we have products (Pr), m-chloroacetophenone in this case. So, in order to switch your learning style from passive looking at this reaction and trying to remember it, take one of those aspects of the reaction, say Rg, and remove it. Then you get:
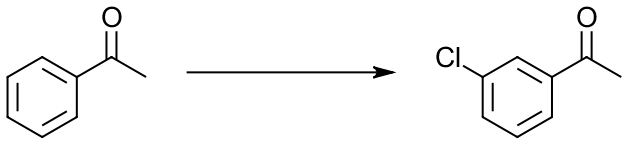
On the other side of your flash card, put the reagents that you didn’t put in the reaction itself. This way, when you look at the reaction, you will have to think what reagents you need to use to accomplish it. If, say, you eliminate the product:
You’ll have to analyze your SM and Rg to find the product, which will be on the back side of the card if you get stuck. This way, you can make three cards for each reaction but all three will be different (one missing SM, one missing Rg, and one missing Pr) and make you actively think about the reaction every time you look at it. Give it a try and let me now how you like this way to use your flash-cards.