Spectroscopy: Fine Details Matter!
When it comes to spectroscopy and solving spectroscopy problems (finding the structure corresponding to the spectrum), one would usually approach it in a sequence of steps. First, I’d look at the molecular formula (or calculate one from the mass-spec) to get the HDI (Hydrogen Deficiency Index or Degree of Unsaturation). That might give some hints on the structural motifs in the molecule. For instance, the HDI = 4 often hints towards the presence of an aromatic ring. Next, I’d look at the IR spectrum to see if there are any clear indications of functional groups in the molecule. Then, I look at the NMR to see what sort of hydrogens and carbons we have in the molecule. Carbon spectrum often helps to identify the presence of symmetry planes in the molecule and see the “hidden” carbons like carbonyls that have no hydrogens attached to them. The hydrogen spectrum helps to find the structural motifs by calculating the number of neighboring hydrogens by applying the “n+1 rule” to the peaks. These steps usually are sufficient to put together the structure. So, let’s look at this problem:
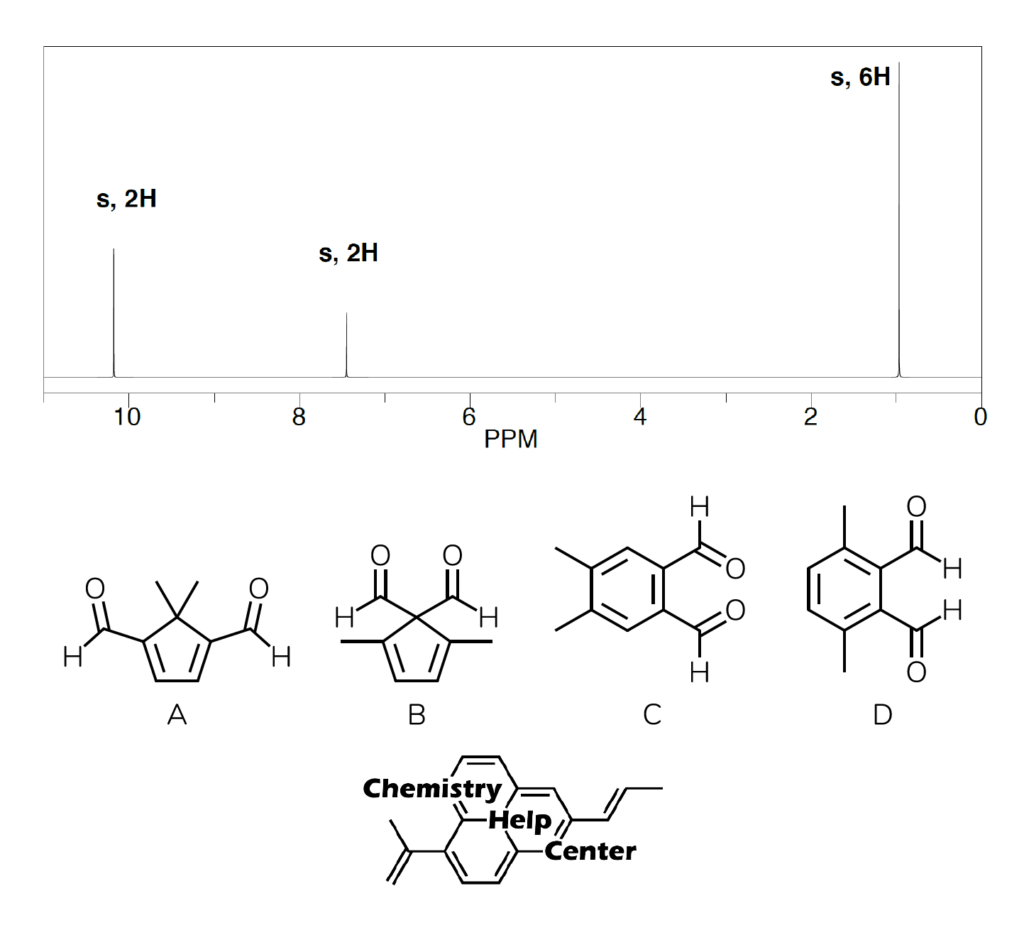
The substitution pattern indicates that we have two -CH3 groups that are aliphatic in nature. Then, we have two hydrogens as one singlet signal in the aromatic region. This indicates that both hydrogens are chemically equivalent and have no neighbors to split with. Finally, we have two hydrogens in the down-field region of the spectrum indicating the presence of two chemically identical aldehyde groups. Thus, a careful reader would go for the answers C or D, and will be absolutely incorrect to do so. Just because the hydrogen shows a signal in the aromatic region, does not mean that it is the aromatic compound. So, what’s the deal?
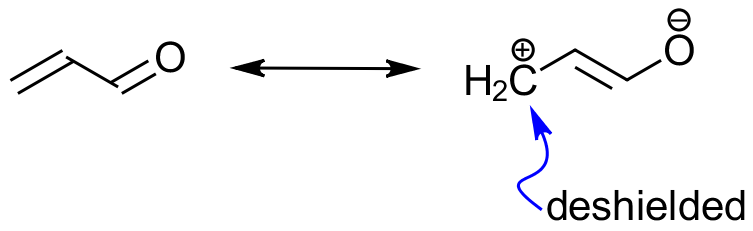
The resonance structures of a molecule can explain the not-so-obvious shielding or de-shielding effects that hydrogens might be experiencing. So, while normally we would expect to see hydrogens on an sp2-hybridized carbon of a double bond at around 4-6 ppm, the conjugated EWG can deshield a β-hydrogen and move it further to the down-filed.
So, the resonance-based deshielding in this case “pulls” the hydrogen to 7-8 ppm, which is the aromatic region. This observation helps us to eliminate the choice B because the aldehyde groups are not conjugated to the alkene and thus won’t cause additional deshileding of the hydrogens on the alkene.
The further analysis of the signals reveal, that the position of the methyl signal is very informative too. It is in the low-ppm aliphatic region which suggests that it must be attached to a non-EWG group, such as an sp3-hybridized carbon plus the shielding effect of the π-electron cloud magnetic field (similar effect to terminal alkynes or large annulenes) lowers the ppm even further. Which disqualifies the options C and D, as those methyl groups would be shifted more down-filed at around 2 ppm. This means, that the correct structure is A!
Would you have given the same answer if you saw this question on your test? Let me know in the comments below!